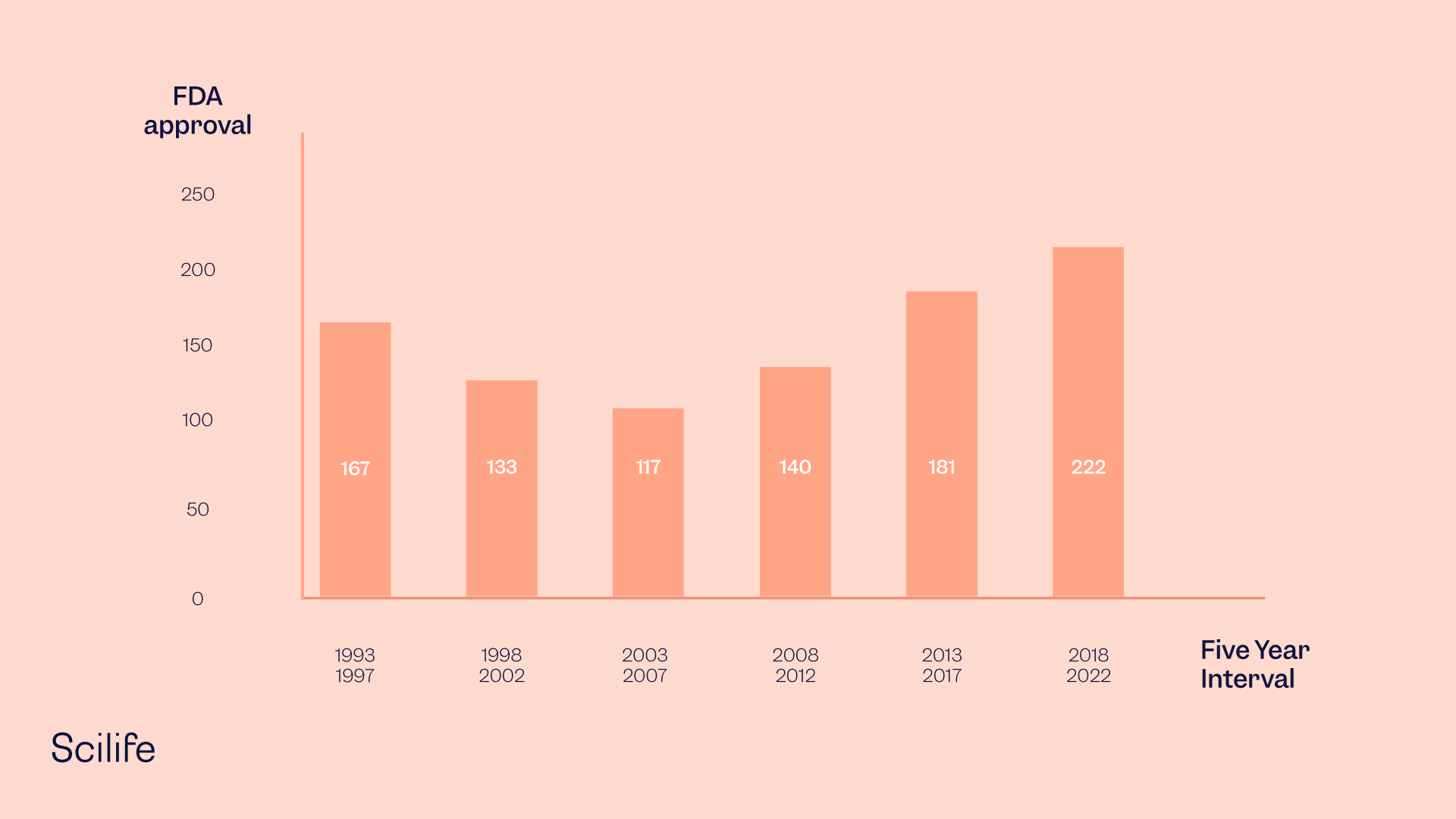
39 fda structured product labels
Structured Product Labeling Resources | FDA Aug 17, 2022 · The Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information. FDA Label Search The drug labels and other drug-specific information on this Web site represent the most recent drug listing information companies have submitted to the Food and Drug Administration (FDA). (See 21 CFR part 207.) The drug labeling and other information has been reformatted to make it easier to read but its content has neither been altered nor verified by FDA. The drug labeling on …
Recognized Consensus Standards - Food and Drug Administration Medical electrical equipment - Radiation dose documentation - Part 1: Radiation dose structured reports for radiography and radioscopy: 07/15/2019: Materials: 8-493: ISO: 13779-2 Third edition 2018-12 : Implants for surgery - Hydroxyapatite - Part 2: Thermally sprayed coatings of hydroxyapatite: 12/23/2019: Physical Medicine: 16-212: ANSI RESNA ...
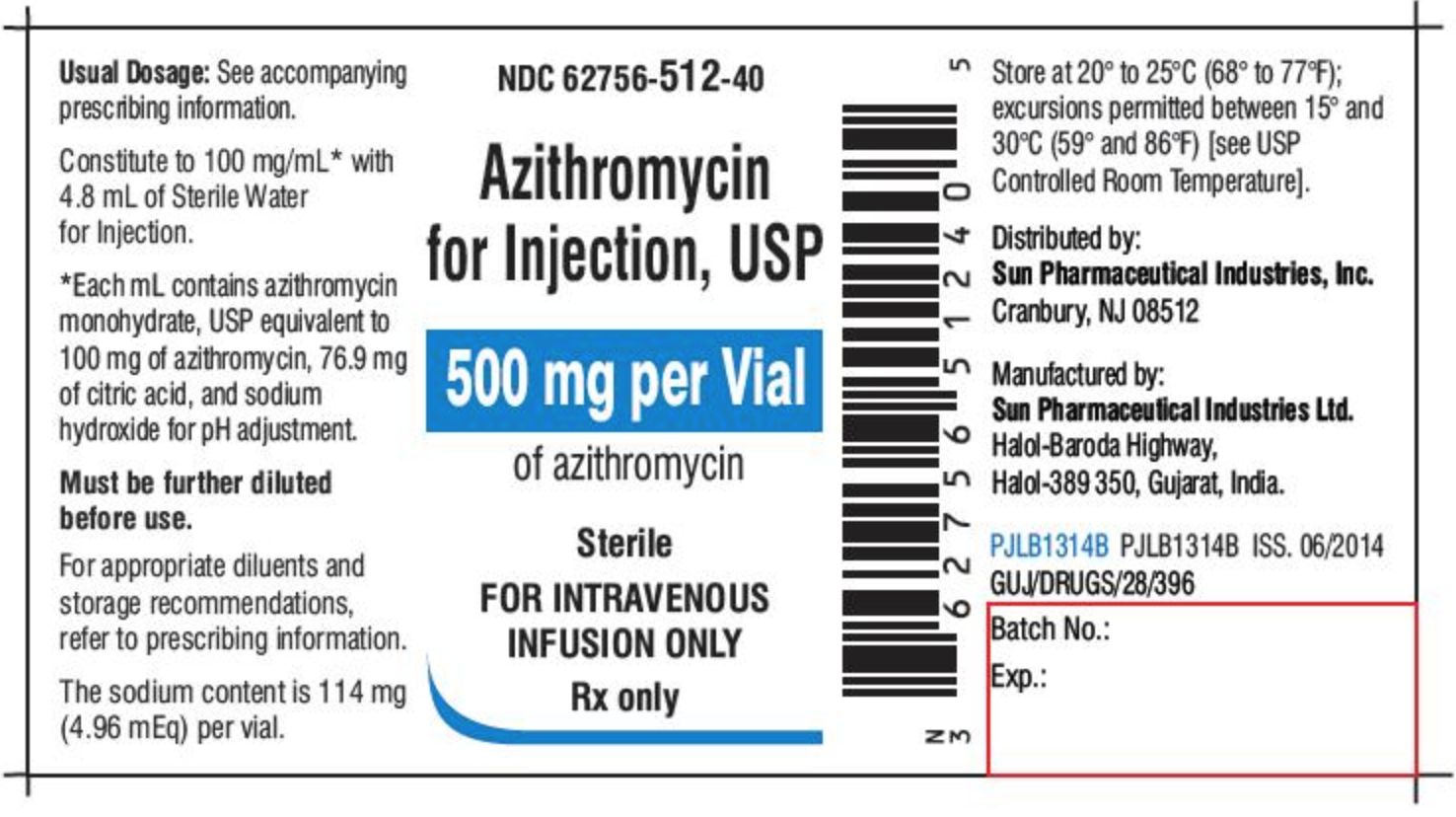
Fda structured product labels
Breast Implants | FDA - U.S. Food and Drug Administration Aug 04, 2022 · Statement from FDA Principal Deputy Commissioner Amy Abernethy, M.D., Ph.D., and Jeff Shuren, M.D., J.D., director of the FDA's Center for Devices and Radiological Health on FDA's new efforts to ... DailyMed - Download All Drug Labels Full Releases. Warning: The full human prescription and OTC archive files, dm_spl_release_human_rx.zip and dm_spl_release_human_otc.zip, are no longer available due to size considerations.Instead, these archives have been split into multiple parts. The remainder archive files consist of bulk ingredient labels, vaccine labels, and some labels for medical … DailyMed 15.09.2021 · The National Library of Medicine (NLM)’s DailyMed searchable database provides the most recent labeling submitted to the Food and Drug Administration (FDA) by companies and currently in use (i.e., "in use" labeling). DailyMed contains labeling for prescription and nonprescription drugs for human and animal use, and for additional products such as medical …
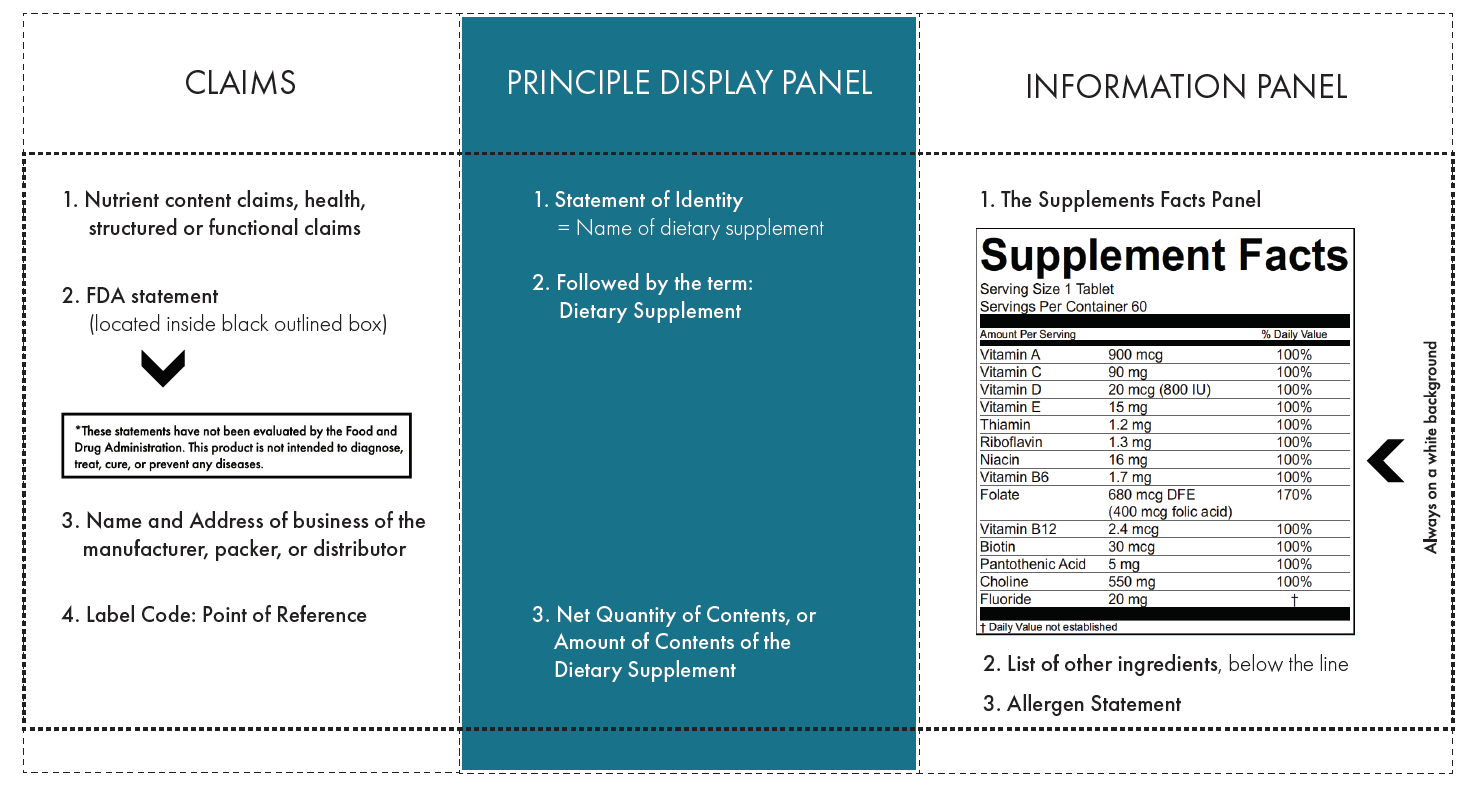
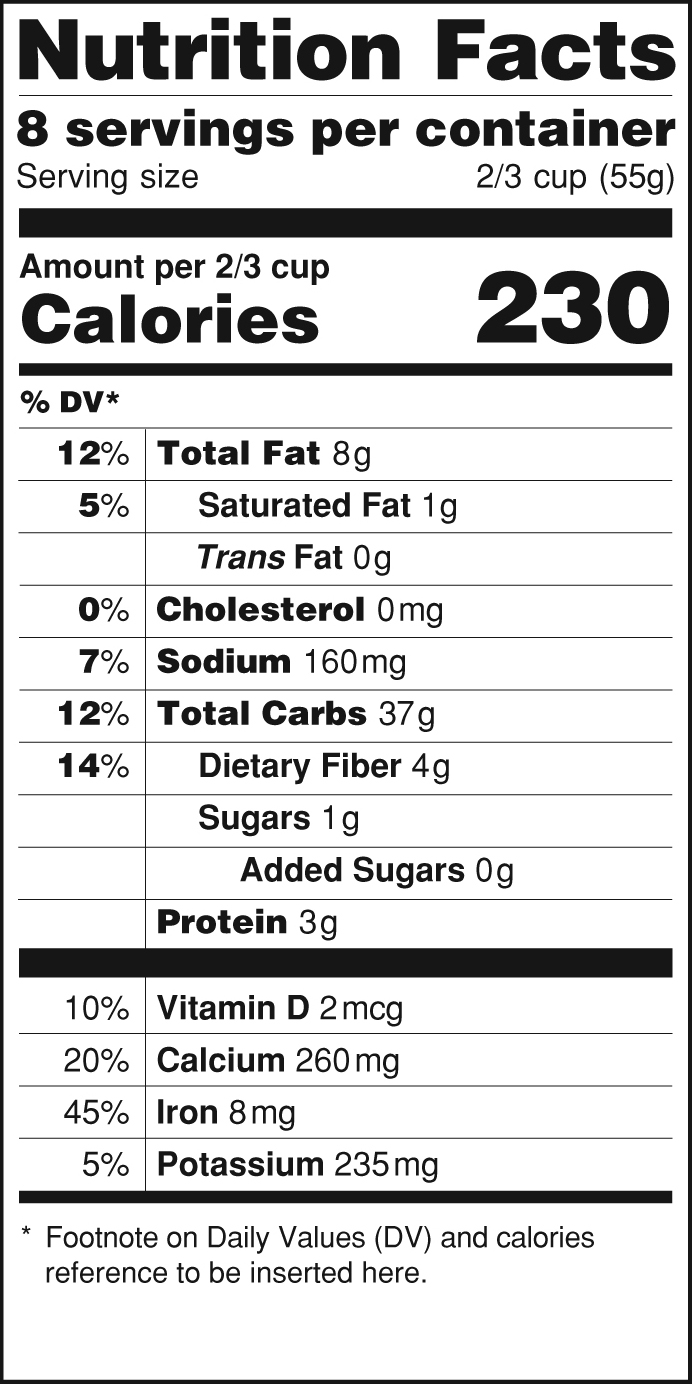
Fda structured product labels. CODE-EHR best practice framework for the use of structured … 29.08.2022 · Big data is central to new developments in global clinical science aiming to improve the lives of patients. Technological advances have led to the routine use of structured electronic healthcare records with the potential to address key gaps in clinical evidence. The covid-19 pandemic has demonstrated the potential of big data and related analytics, but also important … Reed Tech | Best-In-Class Information-Based Solutions and Services 26.09.2022 · Risk Evaluation and Mitigation Strategies in Structured Product Labeling Format & Additional Pharma Annual Deadlines This webinar recording takes a complete look at the annual deadlines that pharmaceutical companies are required to fulfill to become and remain compliant with the US Food and Drug Administration (FDA), with a special focus on the new REMS in SPL … IIS COVID-19 Vaccine Related Code | CDC The following vaccines and associated tradenames have been approved by the FDA under BLA License. They are listed separately because while they may represent the same formulations as the EUA authorized and labeled products listed above, the NDCs listed with the new BLA licensed tradenames in the FDA BLA approval or the FDA Structured Product Labels (SPL) are not currently being produced by the ... Amazon.com: MCR Medical CPR Rescue Mask, Adult/Child Pocket ... Statements regarding dietary supplements have not been evaluated by the FDA and are not intended to diagnose, treat, cure, or prevent any disease or health condition. Customer questions & answers See questions and answers Customer reviews. 4.7 out of 5 stars. 4.7 out of 5. 2,977 global ratings. 5 star 81% 4 star 12% 3 star 4% 2 star 1% 1 star 1% How customer reviews and …
DailyMed 15.09.2021 · The National Library of Medicine (NLM)’s DailyMed searchable database provides the most recent labeling submitted to the Food and Drug Administration (FDA) by companies and currently in use (i.e., "in use" labeling). DailyMed contains labeling for prescription and nonprescription drugs for human and animal use, and for additional products such as medical … DailyMed - Download All Drug Labels Full Releases. Warning: The full human prescription and OTC archive files, dm_spl_release_human_rx.zip and dm_spl_release_human_otc.zip, are no longer available due to size considerations.Instead, these archives have been split into multiple parts. The remainder archive files consist of bulk ingredient labels, vaccine labels, and some labels for medical … Breast Implants | FDA - U.S. Food and Drug Administration Aug 04, 2022 · Statement from FDA Principal Deputy Commissioner Amy Abernethy, M.D., Ph.D., and Jeff Shuren, M.D., J.D., director of the FDA's Center for Devices and Radiological Health on FDA's new efforts to ...








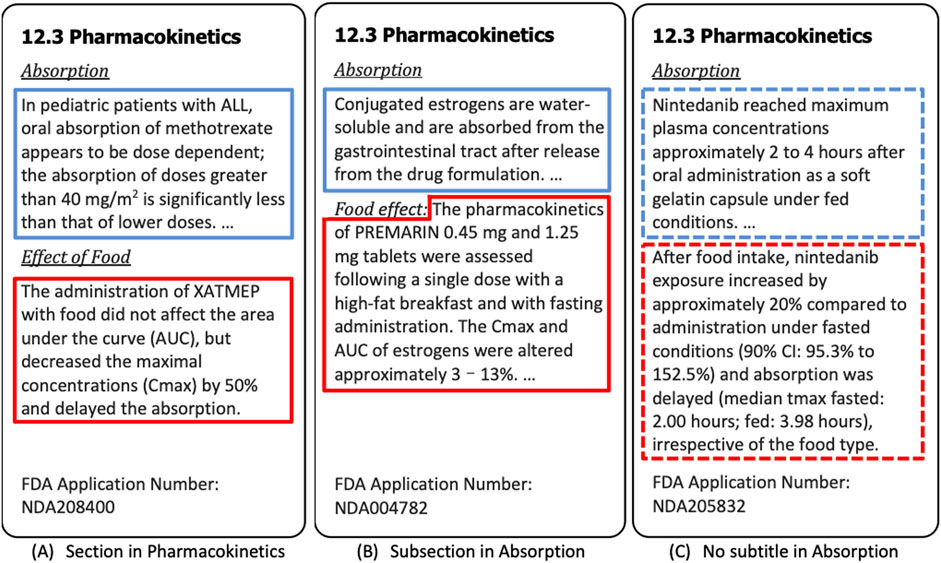





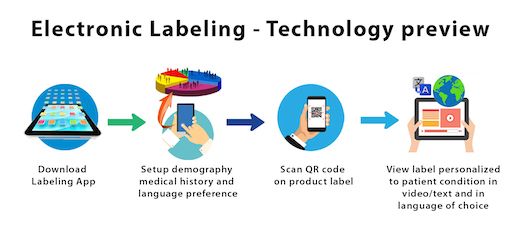


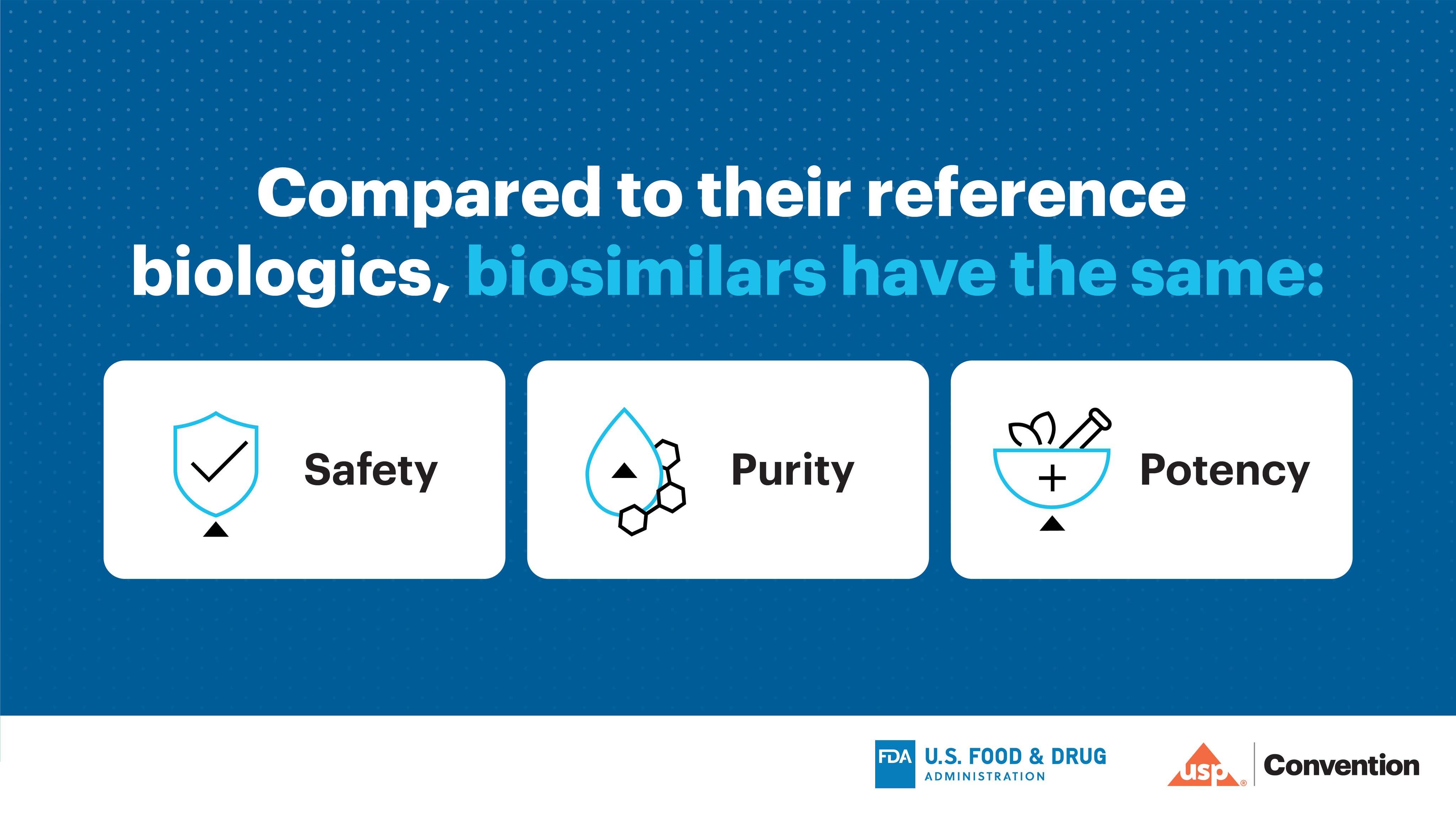



Post a Comment for "39 fda structured product labels"